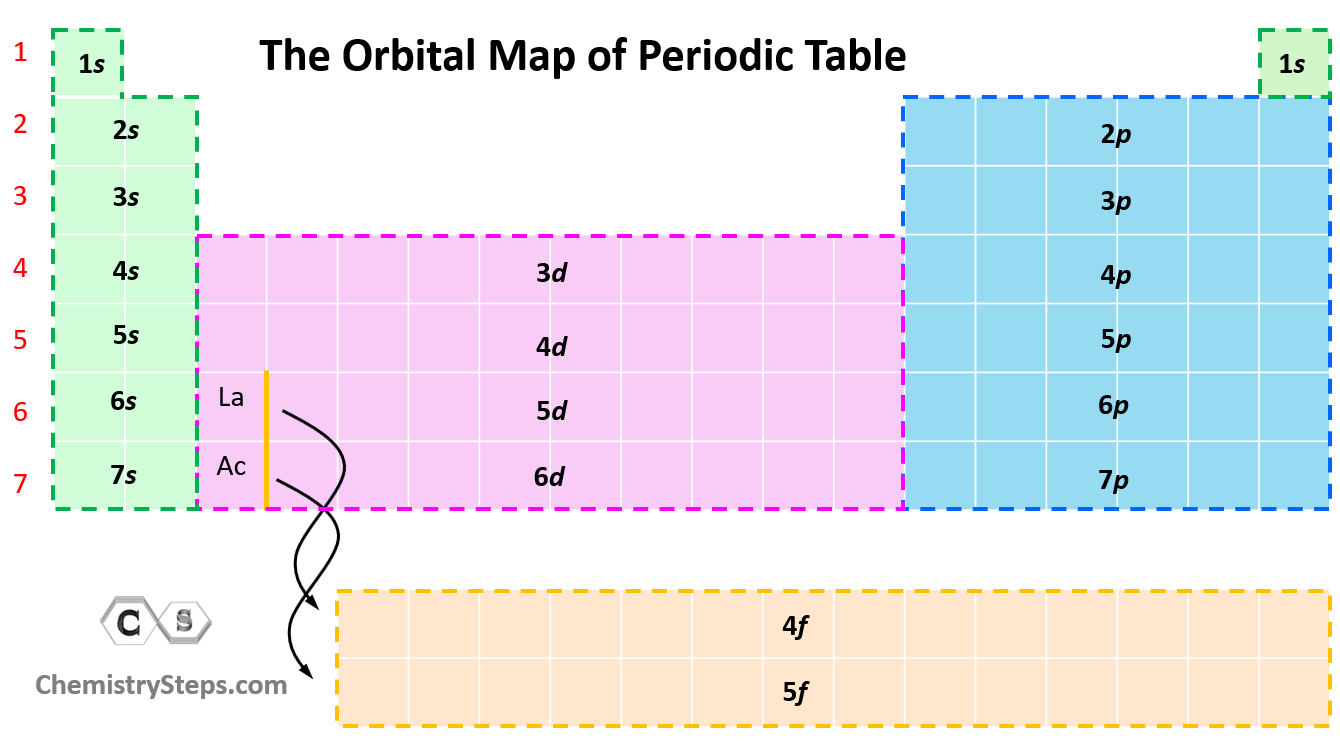
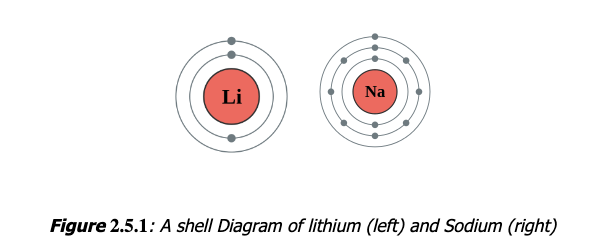
Electron Shell
An electron shell may be thought of as an orbit followed by electrons around an atom nucleus. Because each shell can contain only a fixed number of electrons, each shell is associated with a particular range of electron energy, and thus each shell must fill completely before electrons can be added to an outer shell.
Resources
- https://chem.libretexts.org/Courses/Sacramento_City_College/SCC%3A_Chem_309_-_General_Organic_and_Biochemistry_(Bennett)/Text/02._Atomic_Structure/2.5%3A_Arrangement_of_Electron_(Shell_Model)
- https://general.chemistrysteps.com/orbital-diagrams/
- https://www.expii.com/t/orbital-diagrams-overview-examples-11066