See Charge Model
Electric Charge
History (to help with intuition)
Benjamin Franklin was the person who coined the positive and negative charges. However, these are very subjective terms. Franklin defined a glass rod rubbed with silk as positively charged, while a glass rod rubbed with wool was negatively charged.
Later, when we finally understood what was happening at a microscopic level, we labeled electrons as having a negative charge and protons as having a positive charge to stay consistent which Franklin’s original convention.
- Charges come from the protons and electrons.
- Charge, like mass, is an inherent property of electrons and protons.
- A proton has the same charge as an electron, except in the opposite sign. An electron has a charge of -e, while a proton has a charge of +e, where e is the fundamental unit of charge.
The charge of an object is given by:
2 really important things that you must not confuse:
- A charge of 0 does not mean that there are no charges, so no protons nor electrons. It simply means that the net charge is 0. For instance, an object with 1 000 000 protons and 1 000 000 electrons has a charge of 0.
- An object gains charge not by gaining protons, but by losing electrons. Protons are very tightly bound to the nucleus, so they cannot be added to or removed from atoms. Electrons, however, are bound loosely and can be removed easily.
The Origin of Why Electrons are Negative
Remember two seconds ago when I talked about how it was because of Benjamin Franklin that when we discovered electrons and protons, we labeled electrons as negative while protons were positive? Here is why exactly:
- Franklin labeled a glass rod rubbed with silk as positively charged. At a microscopic label, when a glass rod gets rubbed with silk, the piece of silk strips away the electrons from the glass rod and are moved to the piece of silk. As a result, the glass rod has a surplus of protons. Since Franklin labeled this glass rod which had a surplus of protons as positive, protons are thus considered positive.
- When a glass rod is rubbed with wool, the glass rod snatches the electrons off the piece of wool. The glass rod has a surplus of electrons, which Franklin labeled as negative. Consequently, electrons are considered negative.
Intuitively, we should have made the electrons positive, because it is the electrons that move, not protons. Benjamin made the assumption that protons were moving.
How does charged rod pick up paper?
A charged rod picks up pieces of paper by
- Polarizing the atoms in the paper
- Exerting an attractive polarization force on each atom
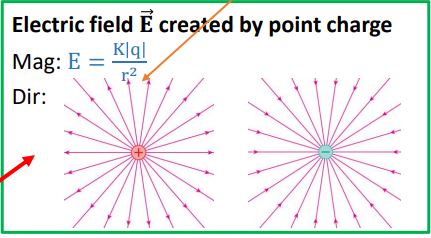
The Field Model
Charges alter a space around them called the Electric Field. We need fields because fields are the agents that are going to allow us to calculate the electric force on a charged particle.
Electric fields move away from positive charges towards negative charges.